Structure-activity Relationships of Diesel Oxidation Catalysts based on Alumina-supported Platinum Nanoparticles
Abstract
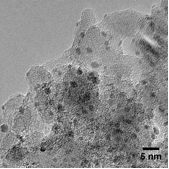
A diesel oxidation catalyst (DOC) is one of the three exhaust aftertreatment catalysts present on modern diesel vehicles. Its function is to catalyse the oxidation of carbon monoxide (CO) and uncombusted fuel to CO2 and water, as well as the oxidation of nitric oxide (NO) to nitrogen dioxide (NO2). The active component of the DOC are platinum nanoparticles dispersed on a ceramic aluminium oxide support, the oxidation taking place on the platinum surface. The aim of the project was to investigate the effect of the particle size on the catalytic activity of the DOC, hence to optimise this catalyst. A series of samples with varying particle sizes was prepared and characterised by transmission electron microscopy (TEM) and hydrogen chemisorption. The activity was measured under mimicked reaction conditions of a diesel exhaust. In situ X-ray absorption spectroscopy (XAS) facilitated information on the platinum oxidation state and coordination number. The results showed that the criteria for optimum conversion of CO is different from that of NO, suggesting different mechanisms for the two reactions. XAS results showed that the platinum was covered with a surface oxide during catalysis of both reactions, showing that this oxide plays a role in the catalysis.
Refreshments will be served after the talk.