Structure-function of plant proteoglycan degrading enzymes
Seminar by Satoshi Kaneko (National Food Research Institute, Tsukuba, Japan)
Abstract:
Arabinogalactan-proteins (AGPs) are an abundant and heterogeneous class of highly
glycosylated hydroxyproline-rich glycoproteins found in higher plants. Glycoside
hydrolases acting on the carbohydrate moieties of AGPs are useful tool for analyzing
AGPs. So far, we have focused on enzymes acting on the β-1,3-β-1,6-galactan backbone,
which is the common structure of heterogeneous AGPs. Galactanases that hydrolyze
β-1,3- or β-1,6-galactosyl linkages are useful tools since the enzymes hydrolyze AGPs
and produce the constituent carbohydrate moieties of AGPs.
We cloned two kinds of galactanases -- exo-β-1,3-galactanase (EC 3.2.1.145) from
Phanerochaete chrysosporium and endo-β-1,6-galactanase (EC 3.2.1.164) from
Trichoderma viride -- and demonstrated that the enzymes were novel and could be
classified as the glycoside hydrolase family 43 (GH43) and family 5 (GH5), respectively.
Structure-function studies of the enzymes are now underway.
Recently, a β-L-arabinopyranosidase was purified from the culture supernatant of
Streptomyces avermitilis, and its corresponding gene was identified. The enzyme could
remove 0.1% and 45% L-arabinose from gum arabic or larch arabinogalactan,
respectively. X-ray crystallographic analysis reveals that the protein had a GH27 catalytic
domain, an antiparallel β-domain containing Greek key motifs, another antiparallel
β-domain forming a jellyroll structure, and a carbohydrate-binding module family 13
domain (Fig. 1). A single amino acid substitution from glutamate to aspartate near the
active site can change the specific to the one of α-D-galactopyranosidase. This is the first
report in which β-L-arabinopyranosidase is classified as a new member of the GH27
family.
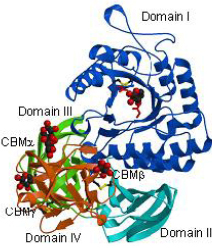
Fig. 1 Overall folding of β-L-arabinopyranosidase
