Bridging the Catalyst Reactivity Gap Between Au and Cu for the Reverse Water Gas Shift Reaction
Authors: Dengxin Yan, Henrik H. Kristoffersen, Ivano E. Castelli, and Jan Rossmeisl
Download databases
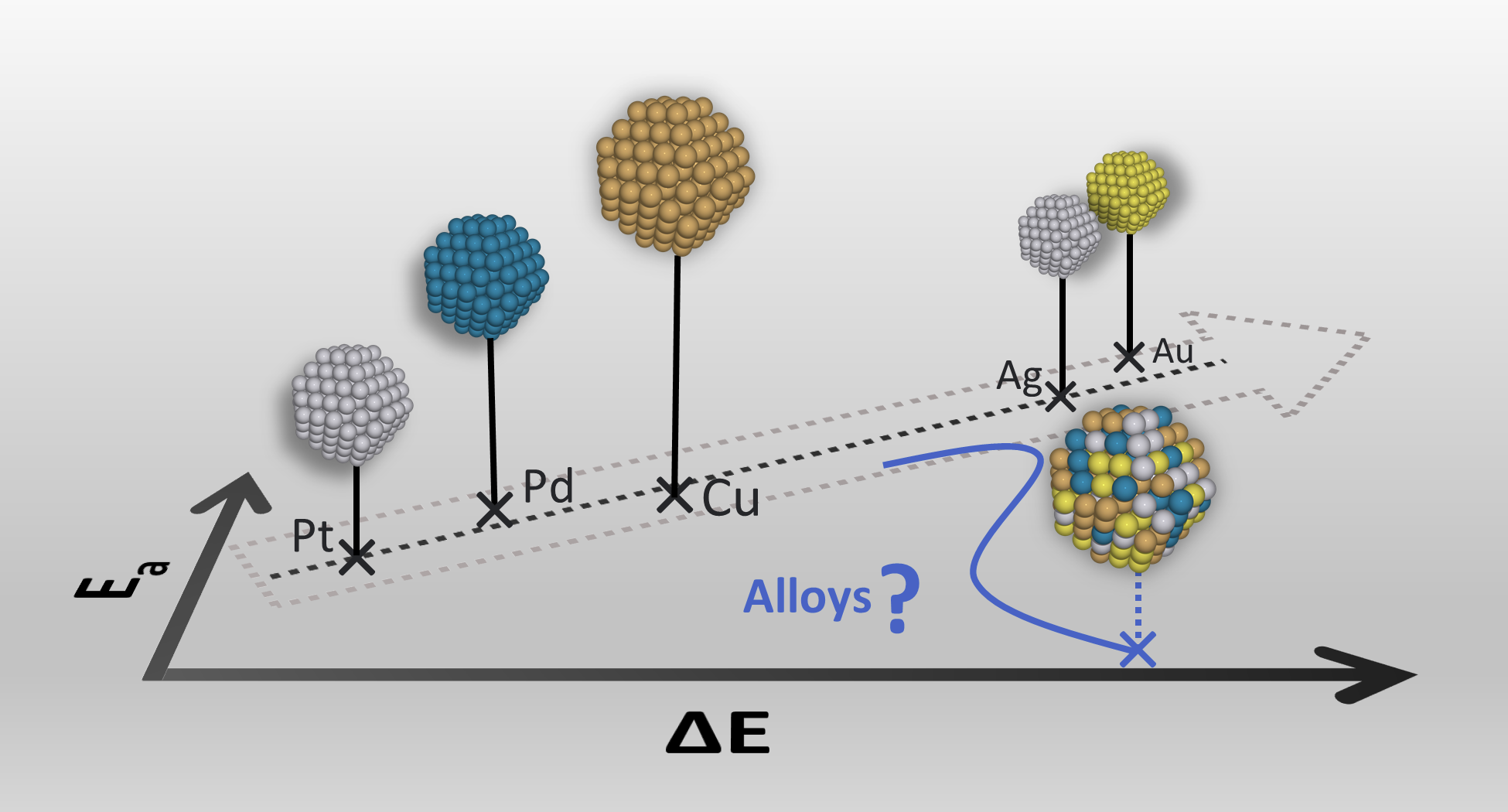
Abstract: The reverse water gas shift reaction (rWGSR) is highly relevant for CO2 utilization in sustainable fuel and chemical production. Both Au and Cu are interesting for rWGSR catalysis, but it turns out that the reactivities of Au and Cu are very different. In this study, we consider alloys made from Au, Ag, Cu, Pt, and Pd to identify surfaces with reactivities for CO2 dissociation in between Cu(111) and Au(111). Additionally, interesting alloy surfaces should have activation energies for CO2 dissociation that are only a little higher than the endothermic reaction energy. We find that certain Cu based alloys with Ag and Au meet these criteria, whereas alloys containing Pt or Pd do not. The low additional cost in activation energy occurs when the transition state and final state are made to look very similar by the choice and placement of the different elements in the surface. Finally, we construct a kinetic model that compares the rate of the rWGSR to the estimated rate of unwanted side reactions (i.e. methane formation or coking) on Ag-Cu alloy surfaces with varying composition and random placement of the Ag and Cu atoms. The thermodynamics favor methane formation over rWGSR, but the model suggest that Ag-Cu alloy surfaces are highly selective for rWGSR.