Climbing the 3D Volcano for the Oxygen Reduction Reaction Using Porphyrin Motifs
Hao Wan, Thomas Mandal Østergaard, Logi Arnarson, and Jan Rossmeisl
H. Wan et al. /ACS Sustainable Chemistry & Engineering Article ASAPDOI: https://pubs.acs.org/doi/abs/10.1021/acssuschemeng.8b04173Description
Here we demonstrate the feasibility of this strategy in a systematic study of diporphyrin molecular catalysts. This class of catalysts contains two metal sites, whose catalytic chemistry can be influenced by ligands. Using density functional theory (DFT), we study the ORR activity as a function of intermetallic distance, metals, and ligands. Several diporphyrin catalysts are identified with a theoretical overpotential of less than 0.3 V. The enhanced catalytic activity is understood as a combination of a geometric effect from the diporphyrin structure and an electronic effect from the choice of metal center and ligand. We propose a strategy to reduce the energy loss and climb the 3D volcano by appropriate design of the geometric and the electronic effects.
The Database
Download databaseKey-value pairs: Description
Given and used in python scripts, but also listed below here.
Key words for metals database:
Molecules: 'Pac', 'DPA', 'DPD'
Metal: 'MnFe','FeFe','FeCo','FeNi','FeCu','CoCo','CoNi', 'CoCu','NiNi', 'CuCu'
ads: 'OOH', 'OH','O', 'molecule'
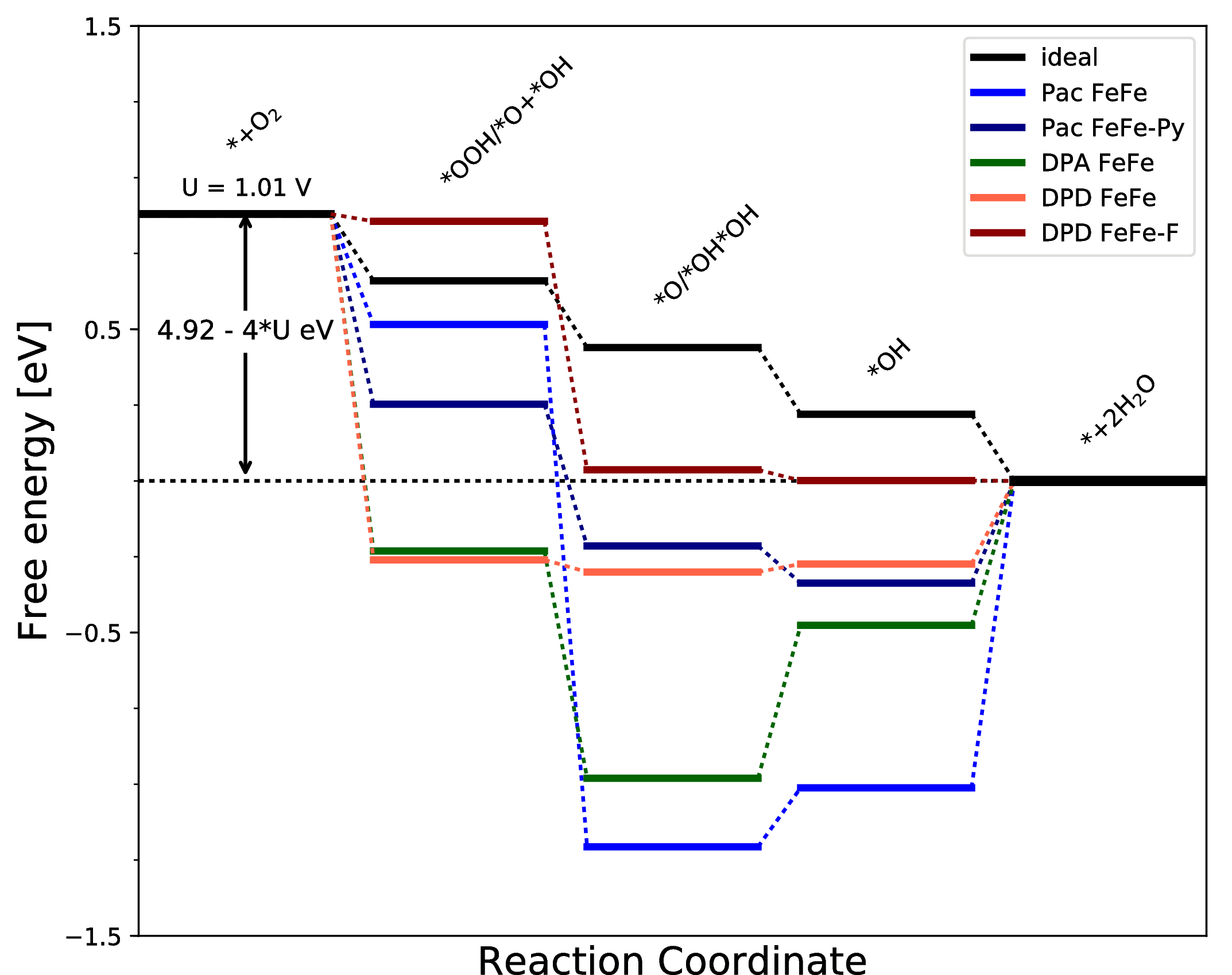
Catalytic cycle for the associative ORR pathway to the left (Pathway 1, black), and the dissociative pathway to the right (Pathway 2, red).
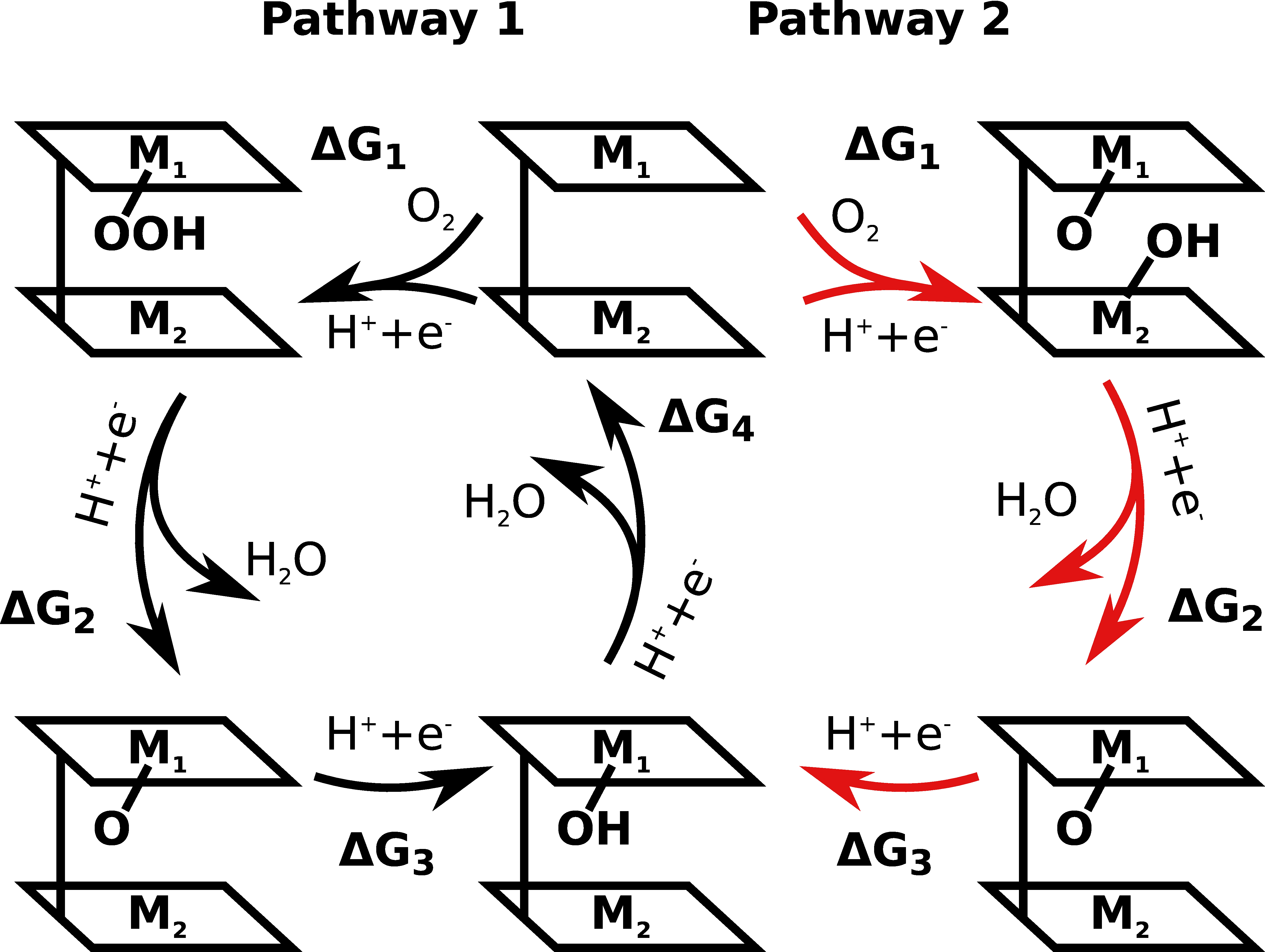
Heat map of the overpotential corresponding to the pair of adsorption energies (*OH and *OOH/*O+*OH).
The script plots the heat map of the overpotential corresponding to the pair of adsorption energies for Pac, DPA and DPD
Download script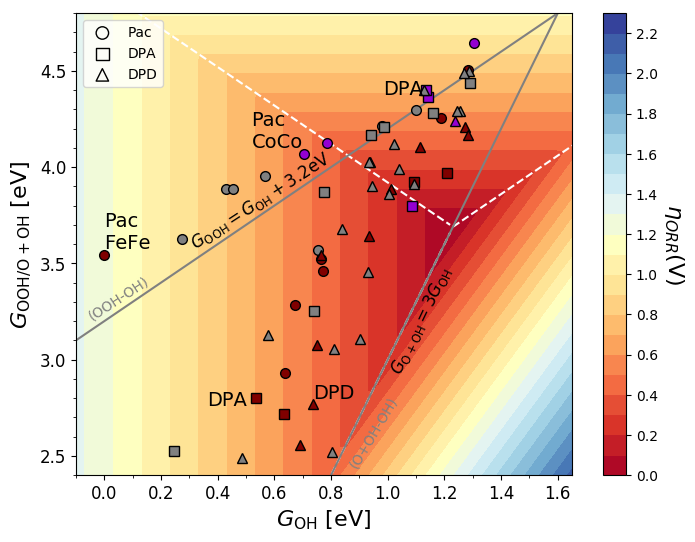
ORR free energy diagram for the different FeFe motifs plotted at U = 1.01 V where all the steps for the DPD FeFe-F catalyst are exergonic.