Electrochemical CO Reduction: A property of the
electrochemical interface
Alexander Bagger, Logi Arnarson, Martin H. Hansen, Eckhard Spohr and Jan Rossmeisl
J. Am. Chem. Soc. 2019, 141, 1506−1514DOI: 10.1021/jacs.8b08839
Description
In this work we investigate how electrolytes affect the electrochemical reduction interface of CO reduction. We present thermodynamically realistic structures of the electrochemical interfaces, by explicit ab initio simulations. We investigate how key CO reduction reaction intermediates are stabilized in different electrolytes, and for different pH. We find that the catalytic trends, previously observed experimentally, can be explained by the interplay between the metal surface and the electrolyte. For the Cu(100) facet with a phosphate buffer electrolyte the energy efficiency is found to be limited by blocking of a phosphate anion, while in alkali hydroxide solutions (MOH, M=Na, K, Cs), OH* intermediates may be present, and at high overpotential the H* coverage limits the reaction. The results provide insight into the electrochemical interface structure, revealing
the limitations for multiple carbon products and offer a direct comparison to experiments.
The Database
Download databases, scripts ect (big .zip file)A selection of figures from the manuscript, can be directly reproduced by downloading the .zip file and running the python scripts.
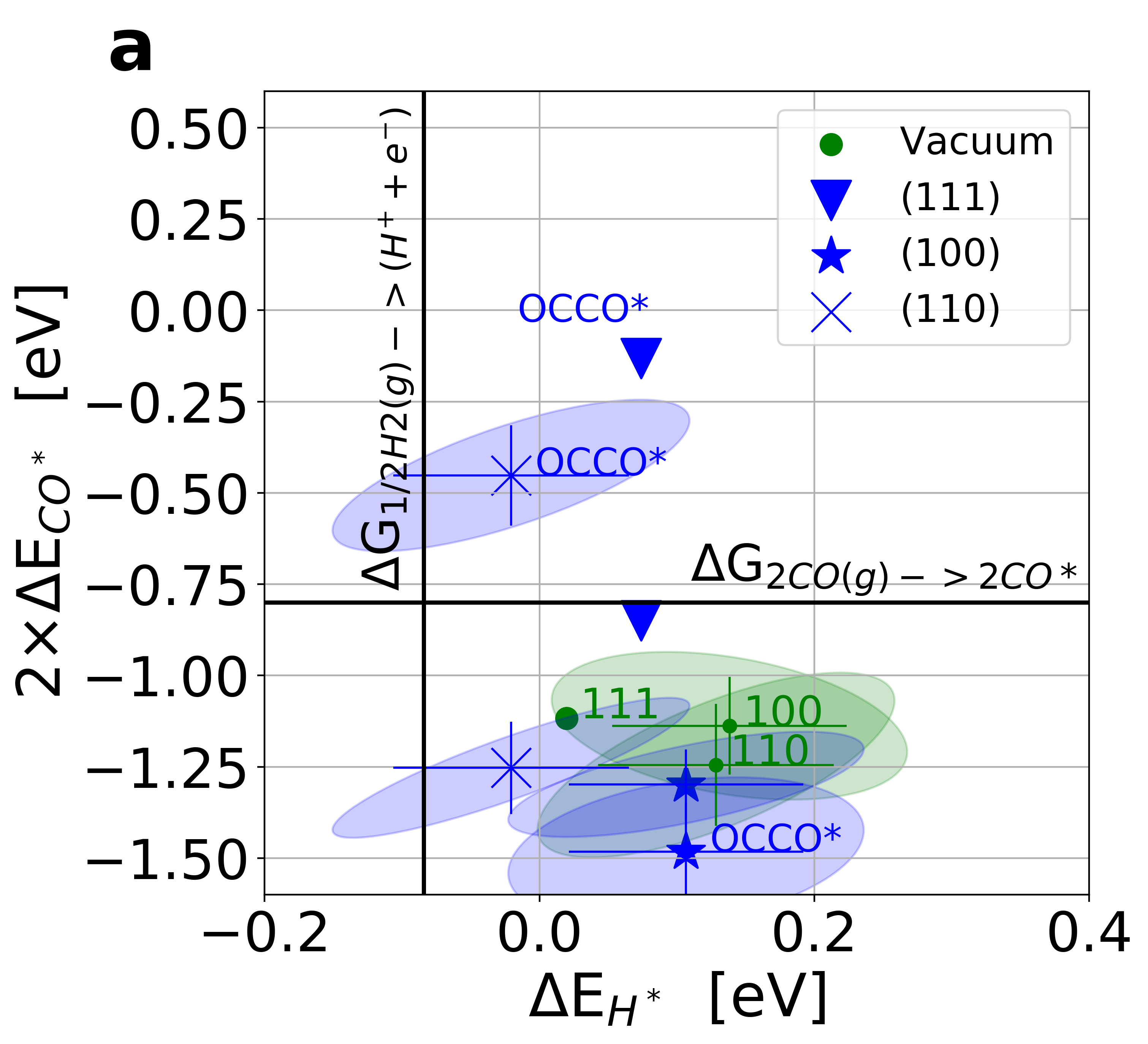
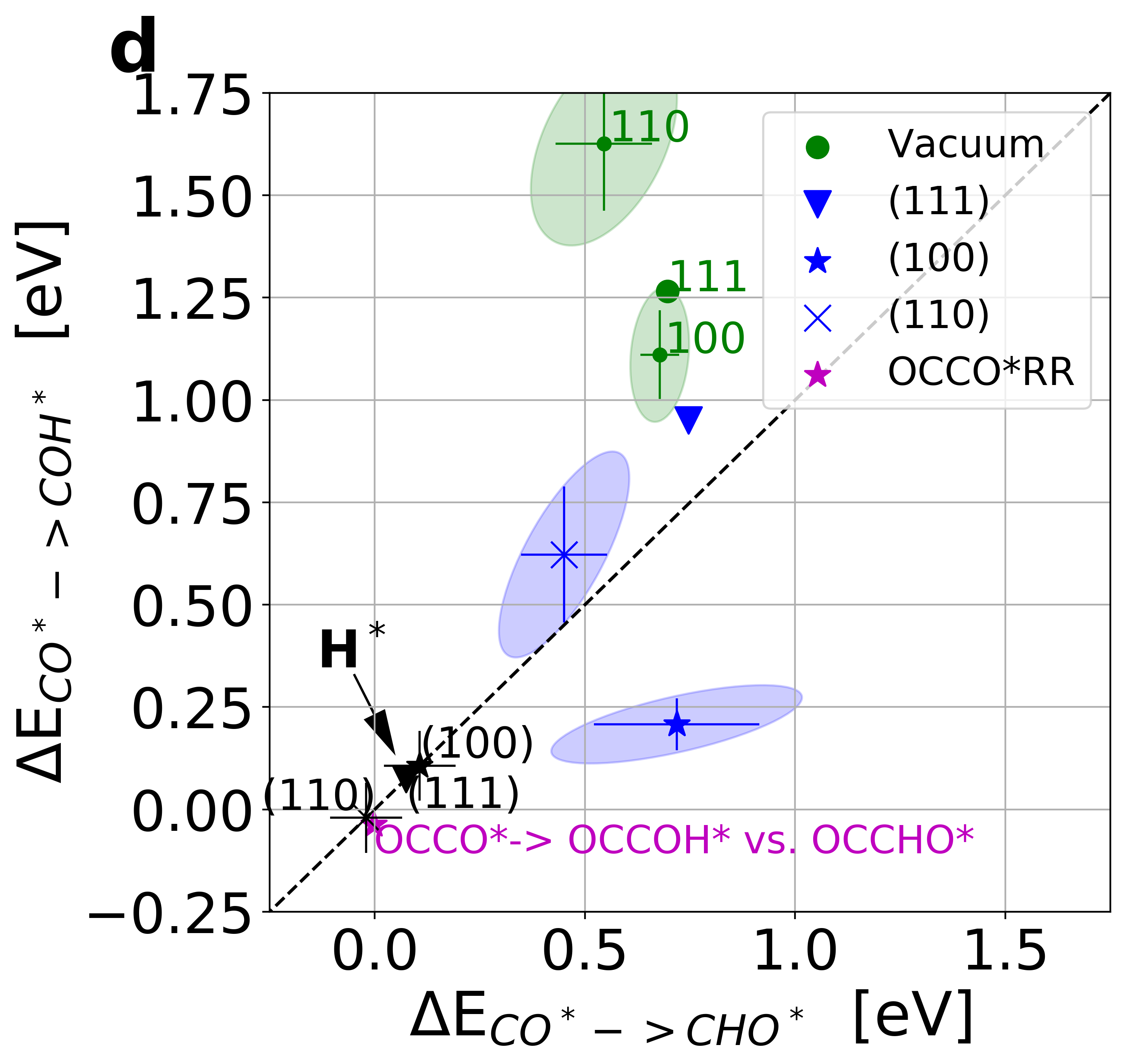
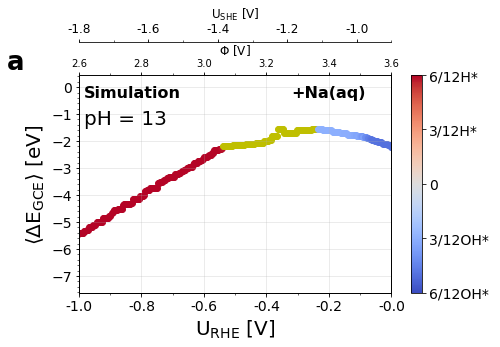
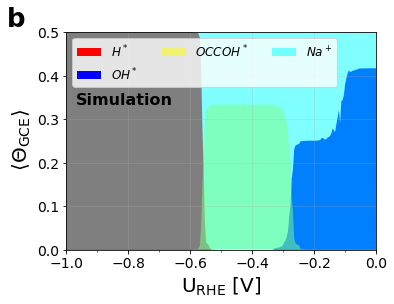
Figure 3
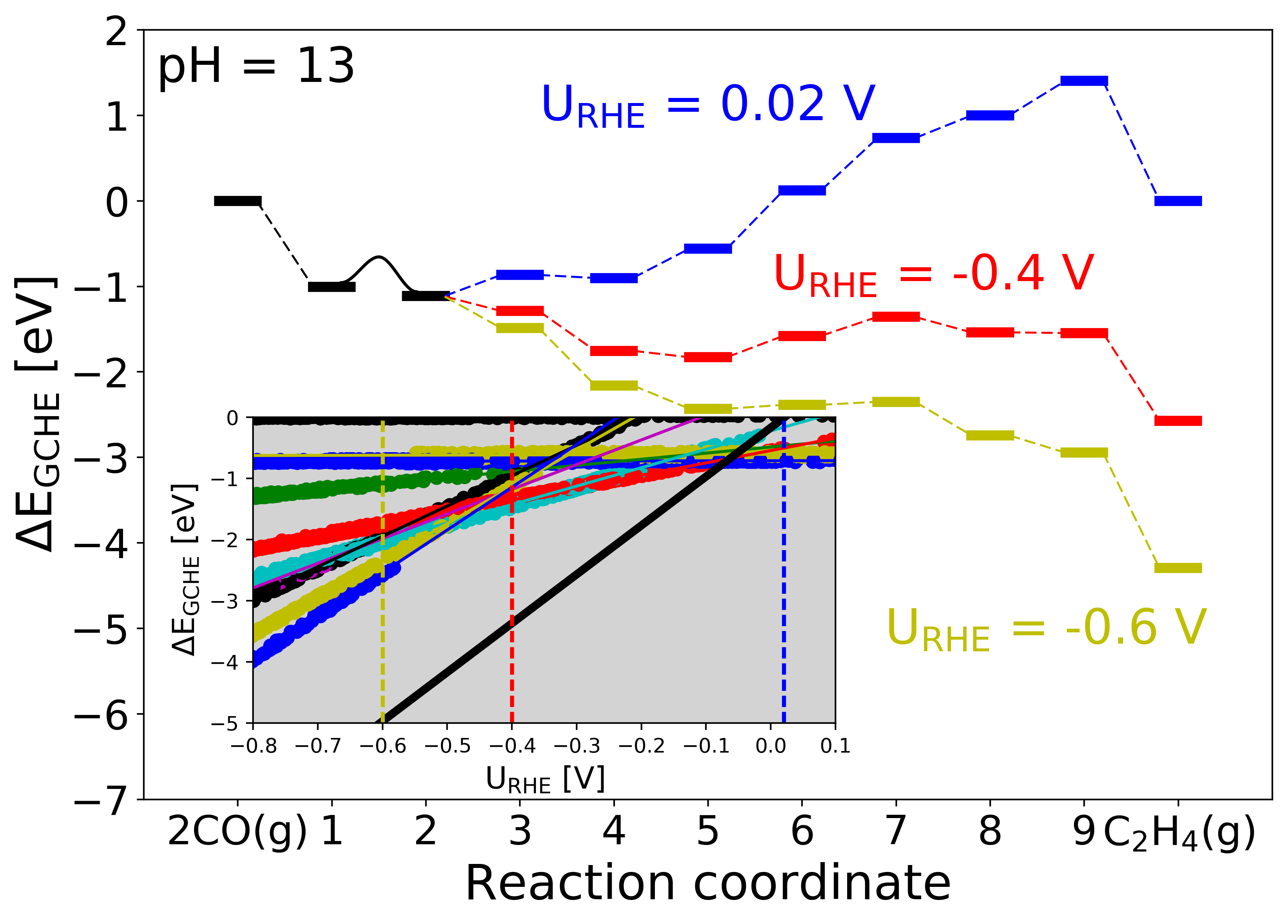
Figure 4
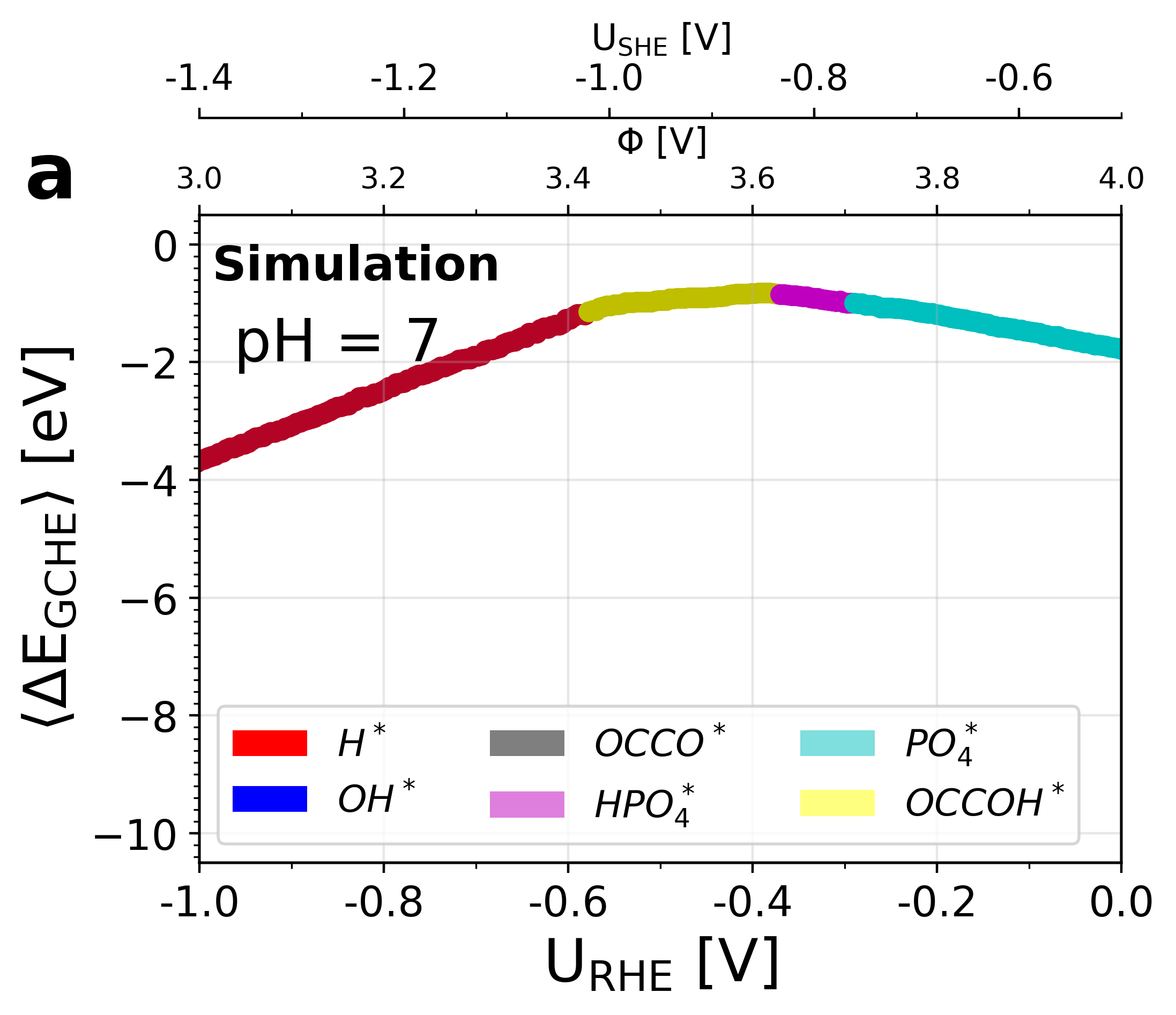
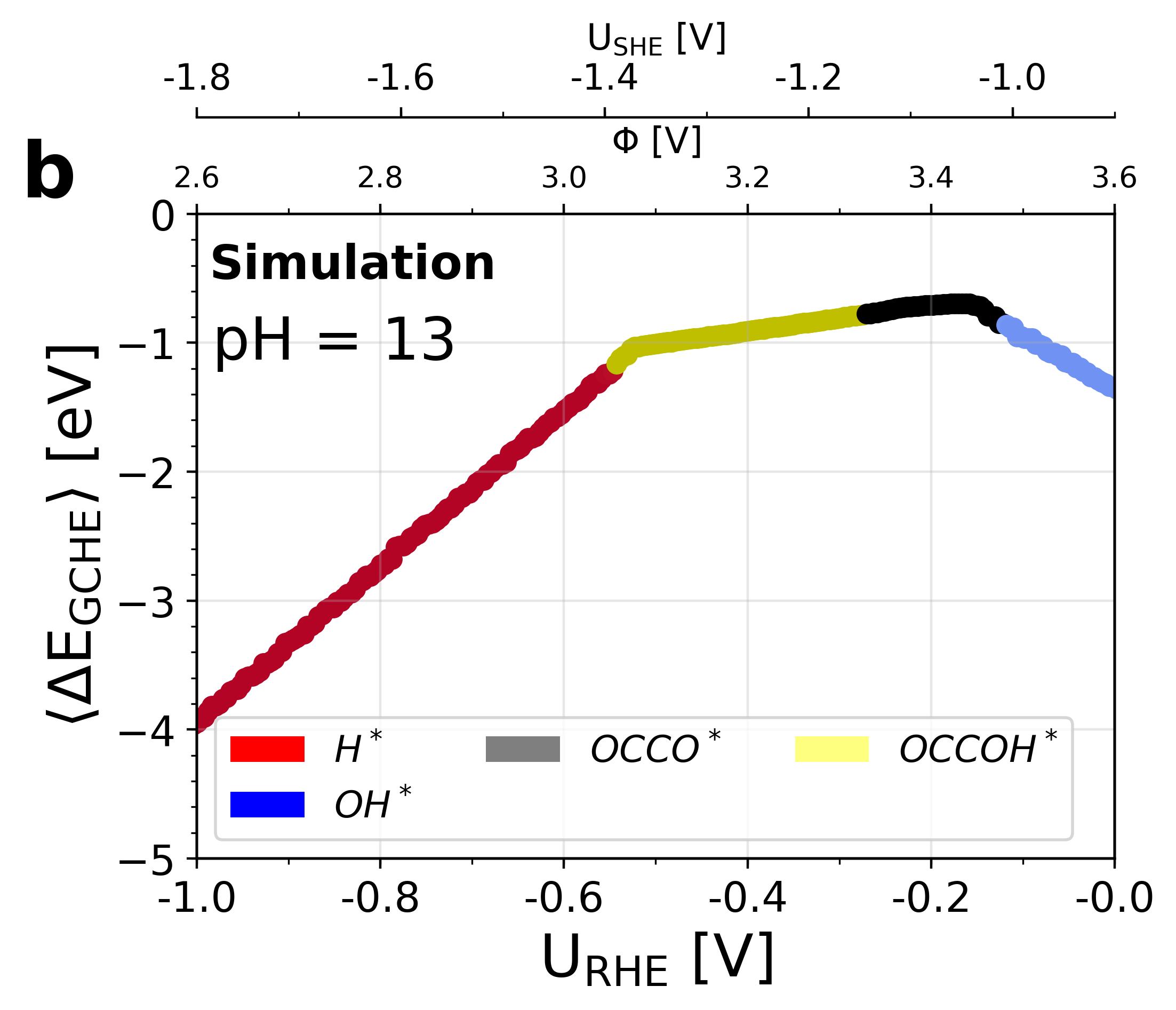
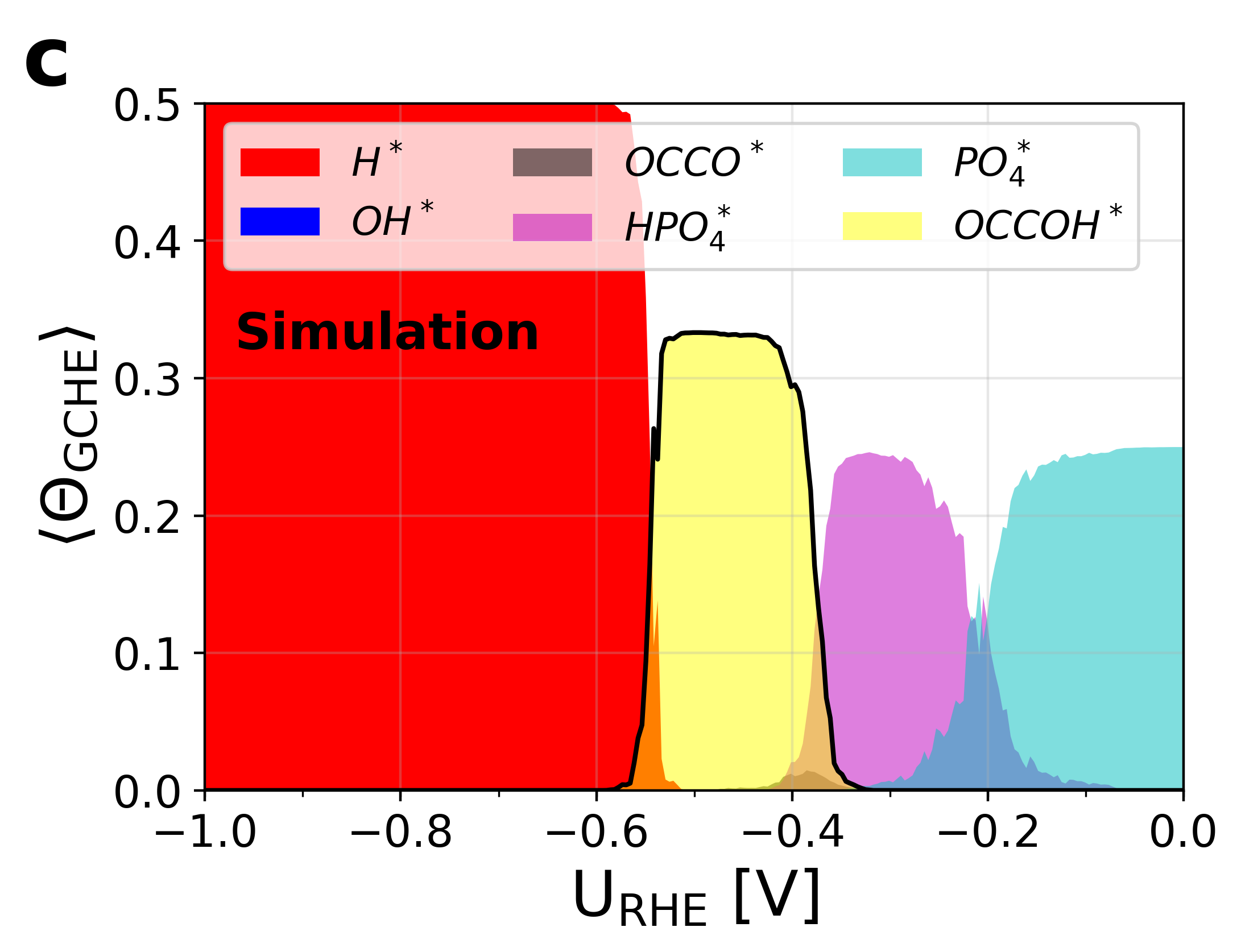
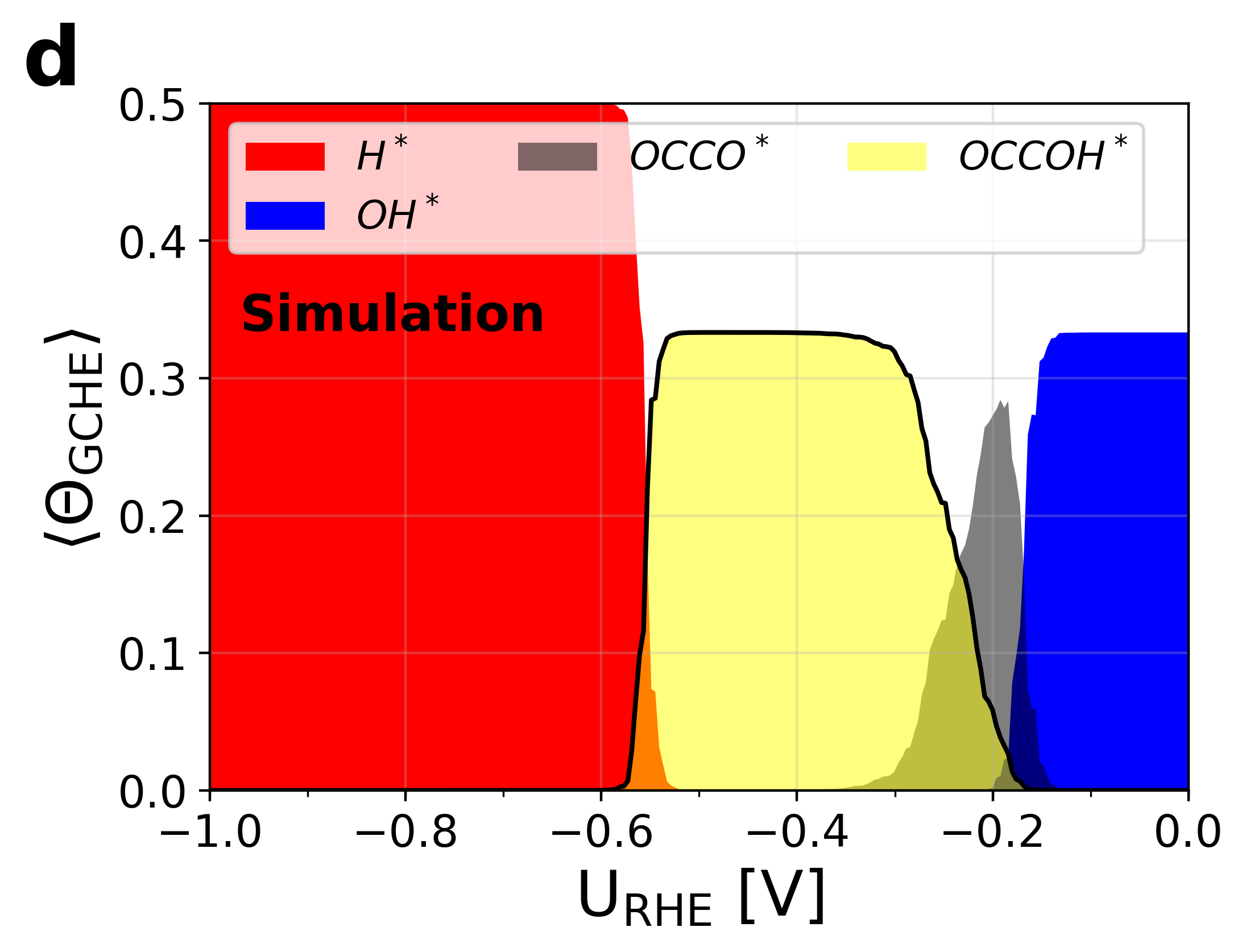
Figure 5
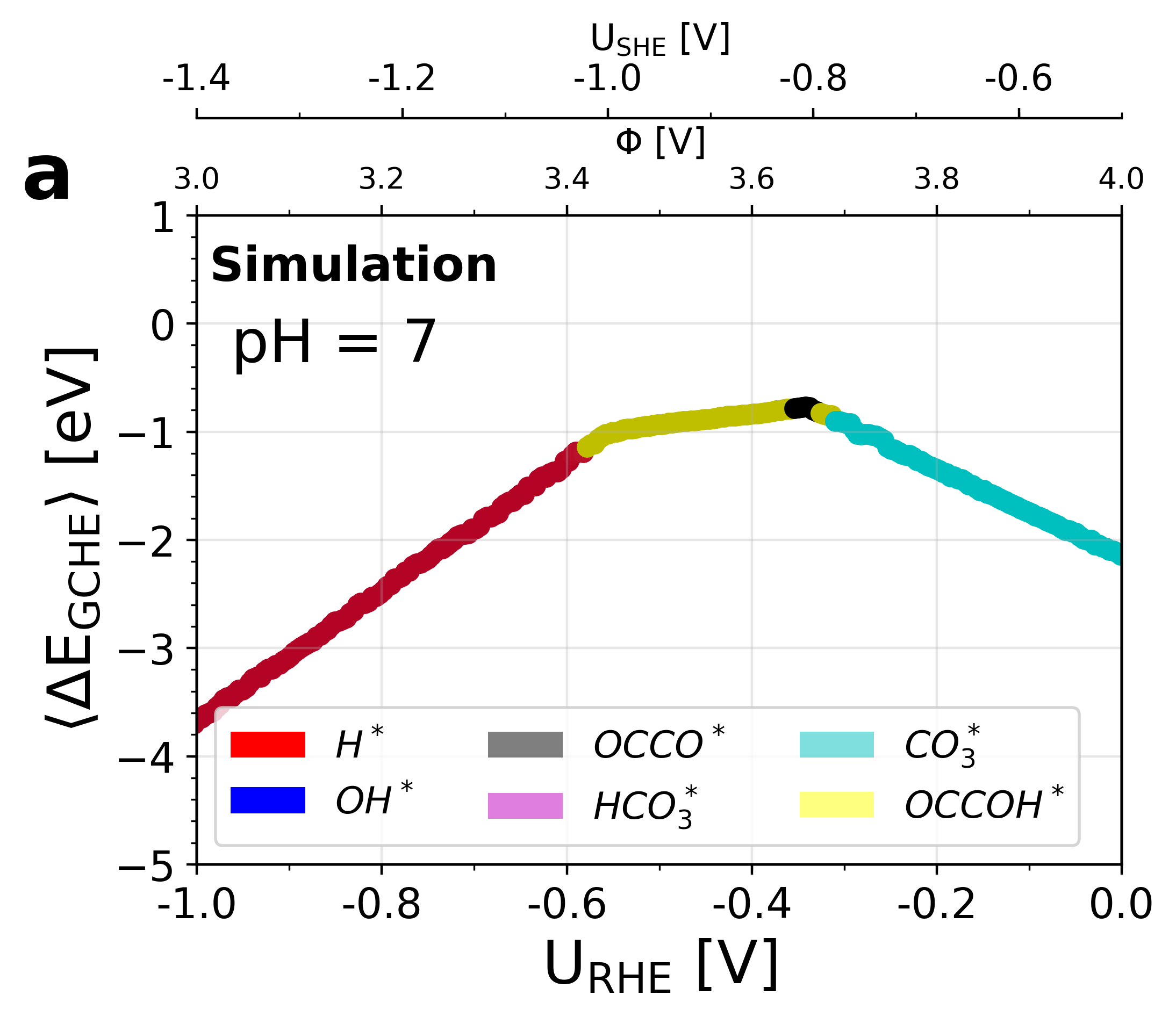
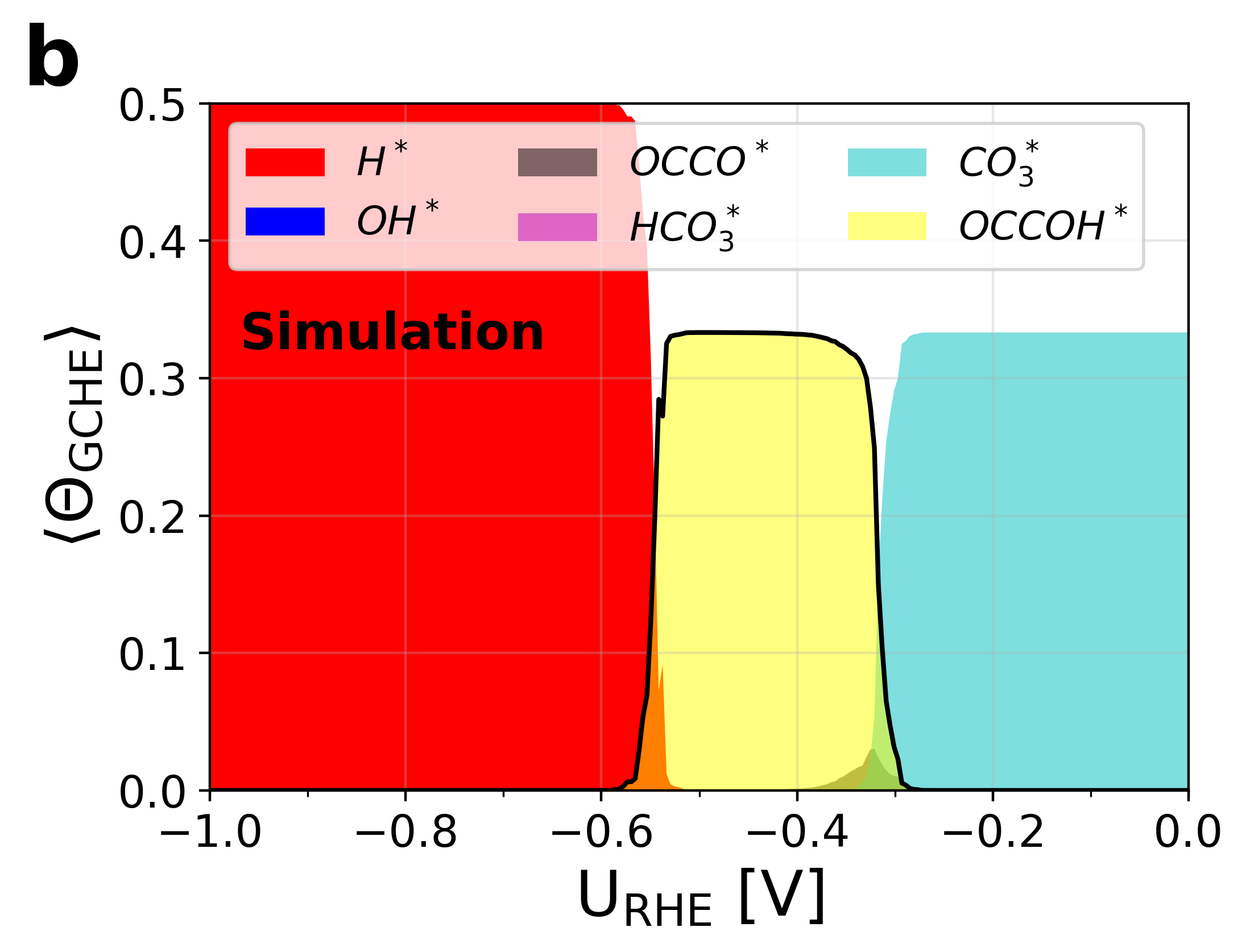
Figure 6
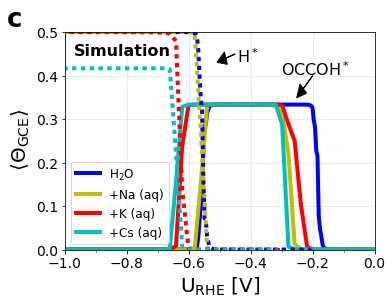
Figure S9