Electrochemical Nitric Oxide Reduction on Metal Surfaces
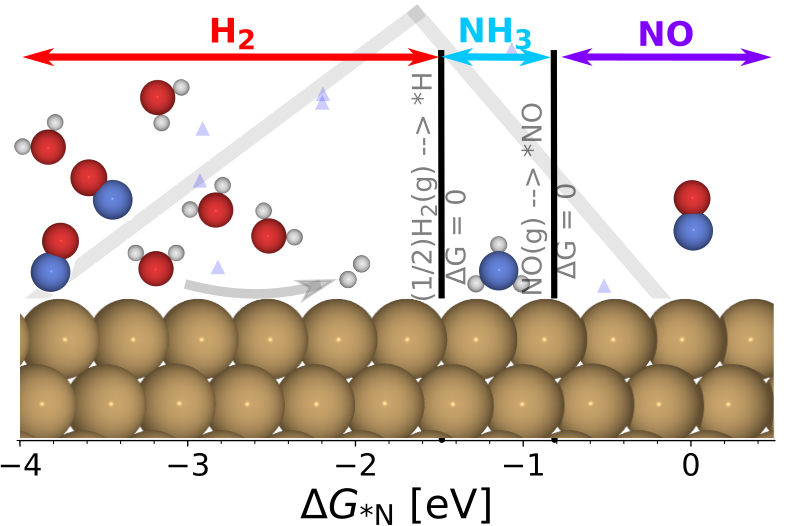
We elucidate selectivity and activity of catalyst for NOxRR. At low potential we classify metals by the binding of ∗NO versus ∗H. Analogous to classifying CO2 reduction by ∗CO vs ∗H, Cu is able to bind ∗NO while not binding ∗H giving rise to a selective NH3 formation. Besides being selective, Cu is active for the reaction found by an activity-volcano. For metals that does not bind NO the reaction stops at NO, similar to CO2 -to-CO. At potential above 0.3 V vs RHE, we speculate a low barrier for N coupling with NO causing N2O formation. The work provide a clear strategy for selectivity and aims to inspire future research on NOxRR.
