Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2
Wen Ju, Alexander Bagger, Guang-Ping Hao, Ana Sofia Varela, Ilya Sinev, Volodymyr Bon, Beatriz Roldan Cuenya, Stefan Kaskel, Jan Rossmeisl and Peter Strasser
Manuscript accepted in nature communicationsDOI: 10.1038/s41467-017-01035-zDescription
In this work, catalytically active M-Nx moieties (M = Mn, Fe, Co, Ni, Cu) are investigated for their electrochemical CO2 reduction reaction towards CO, which demonstrates that Fe-N-C and especially Ni–N–C catalysts rival Au- and Ag-based catalysts. We model the catalytic active M–Nx moieties using density functional theory and correlate the theoretical binding energies with the experiments to give reactivity-selectivity descriptors. This gives an atomic-scale mechanistic understanding of potential-dependent CO and hydrocarbon selectivity from the M–Nx moieties and it provides predictive guidelines for the rational design of selective carbon-based CO2 reduction catalysts
The Data files
Download data, database & scriptKey-value pairs: Description
Given and used in python scripts
The M-Nx catalysts CO2 to CO TOF as a function of potential and compare to found desriptors for each potential region:
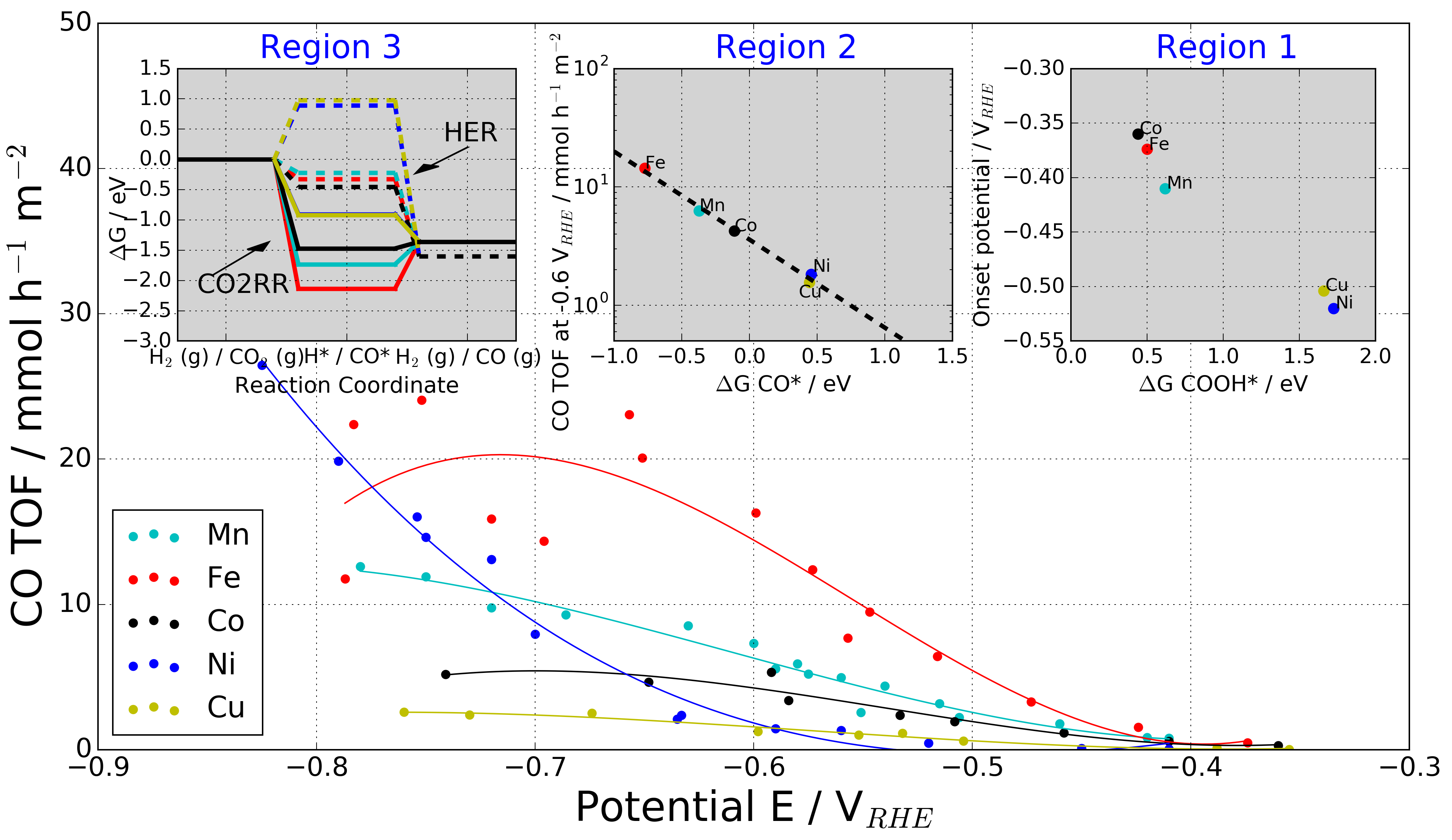
Experimental CO production turnover frequency (TOF) of the M-N-C catalysts versus applied IR-corrected electrode potential. The catalytic reactivity trends split into 3 potential regions with distinctly different rate-determining mechanistic features. Insets: Region 1: Low overpotentials, the experimental onset potentials of CO production correlate with the binding energy of the reaction intermediate COOH*. Region 2: Intermediate over-potentials, CO production TOF at -0.6 V RHE correlates with the free energy of adsorbed CO (CO*). Region 3: High overpotentials, free energy diagrams for the HER (dashed paths) and CO2RR (solid paths) at -0.8 V RHE for each M-N-C catalyst. HER barriers are high for Ni and Cu, while CO2RRis downhill making these materials favorable CO producing catalysts.
