Selectivity and Intrinsic Activity of Functionalized Cu Surfaces: Can the CO2 Reduction Reaction be Improved on Cu?
Cu is the only monometallic catalyst known for producing valuable multi-carbon-based products, such as ethylene and ethanol, from the CO2 reduction reaction (CO2RR). One approach to optimize the activity and selectivity of the metal Cu catalyst is to functionalize the Cu electrode with a molecular modifier.
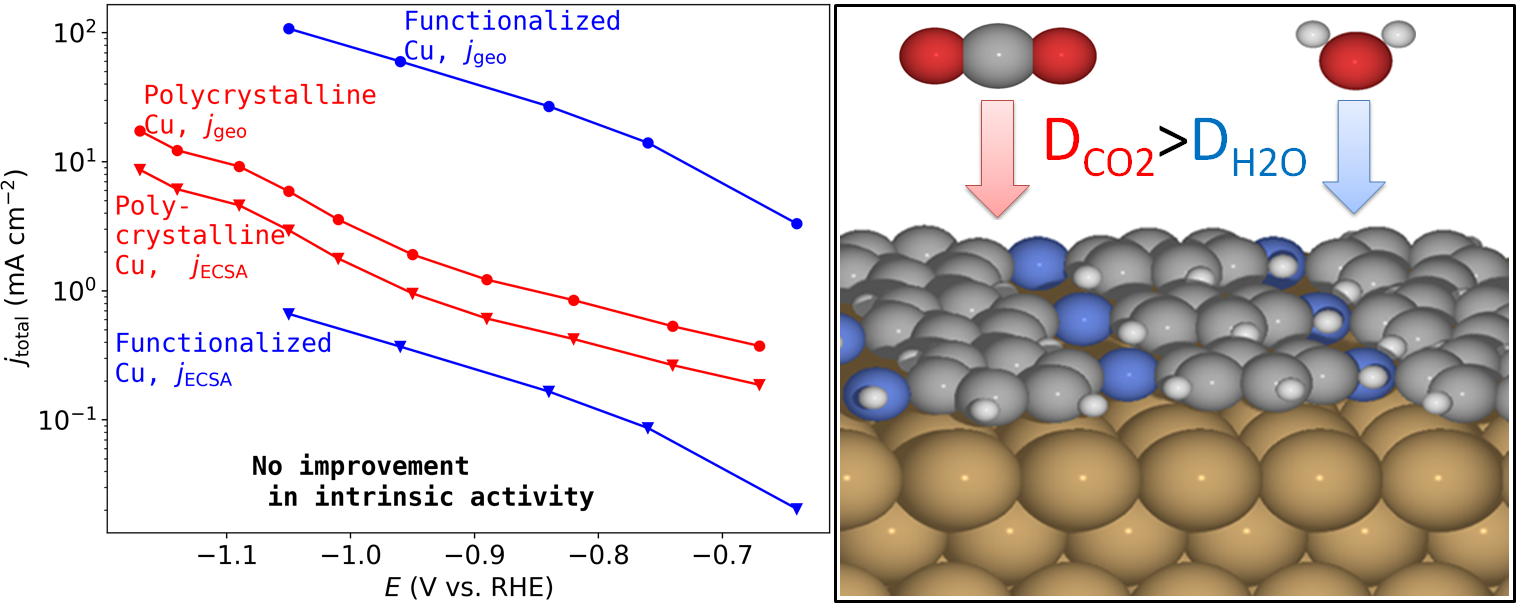
We investigate from a data standpoint whether any reported functionalized Cu catalyst improves the intrinsic activity and/or multi-carbon product selectivity compared to the performance of bare Cu foil and the best single crystal Cu facets. Our analysis shows that the reported increases in activity are due to increased surface roughness and disappear once normalizing with respect to electrochemical surface area. The intrinsic activity generally falls below that of bare Cu foil, both for total and product-specific current, which we attribute to non-selective blocking of active sites by the modifier on the surface. Instead, we show that the modifier allows for easier diffusion of CO2 compared to H2O to the surface, leading to greater selectivity for \ch{CO2RR} and \ch{C2+} products. As such, there is currently not a catalyst for CO2RR that intrinsically outperforms bare Cu.
Download databases and scripts here.
