Steering carbon dioxide reduction toward C–C coupling using copper electrodes modified with porous molecular films
Authors: Siqi Zhao, Oliver Christensen, Zhaozong Sun, Hongqing Liang, Alexander Bagger, KristianTorbensen, PegahNazari, Jeppe Vang Lauritsen, Steen Uttrup Pedersen, JanRossmeisl & Kim Daasbjerg
DOI: https://doi.org/10.1038/s41467-023-36530-z
The full script for the microkinetic model, along with additional figures and videos showing
results for the model, is available here: https://sid.erda.dk/cgi-sid/ls.py?share_id=e3OC2vAbe1.
Abstract:
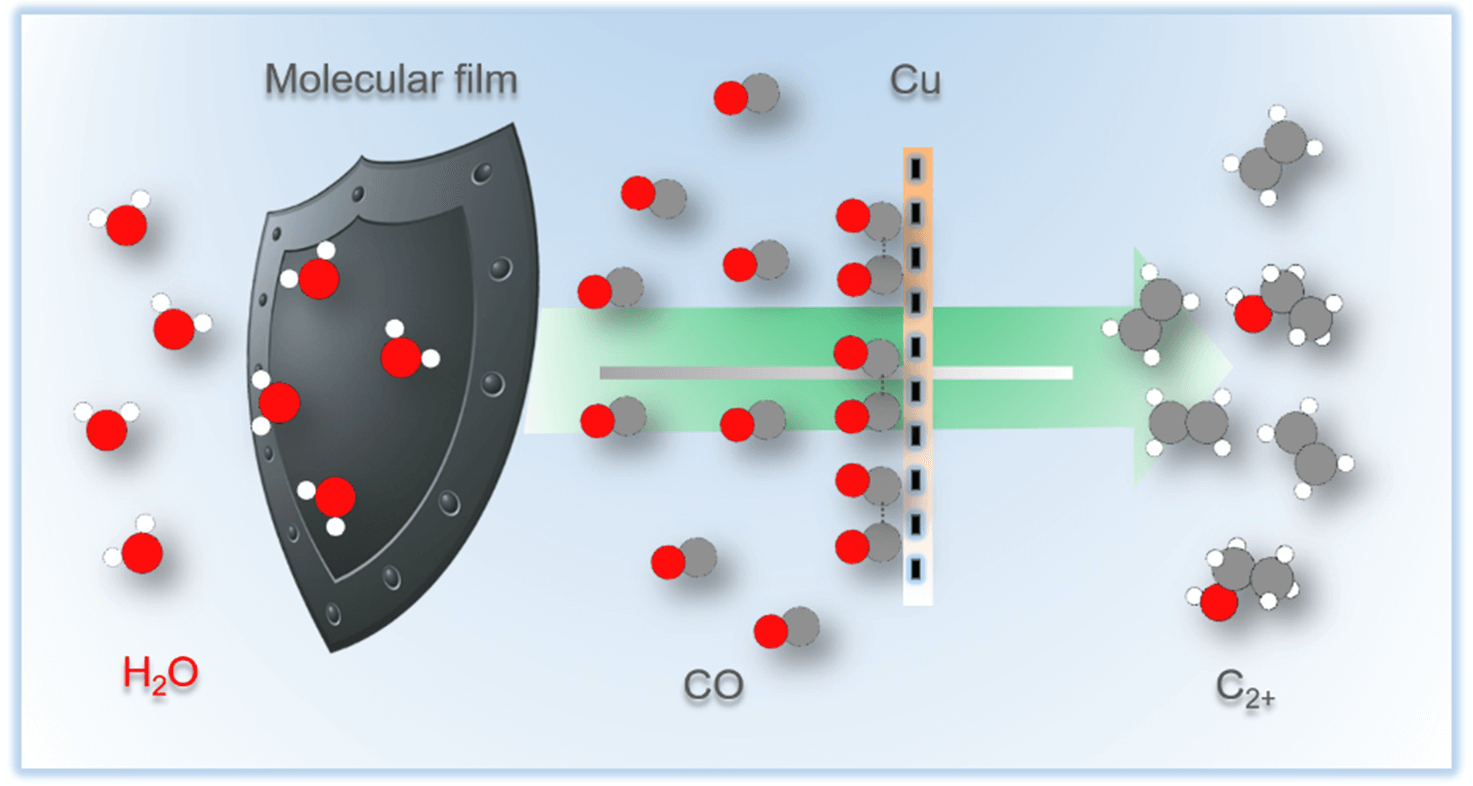
Copper offers unique capability as catalyst for multicarbon compounds production
in the electrochemical carbon dioxide reduction reaction. In lieu of
conventional catalysis alloying with other elements, copper can be modified
with organic molecules to regulate product distribution. Here, we systematically
study to which extent the carbon dioxide reduction is affected by film
thickness and porosity. On a polycrystalline copper electrode, immobilization
of porous bipyridine-based films of varying thicknesses is shown to result in
almost an order of magnitude enhancement of the intrinsic current density
pertaining to ethylene formation while multicarbon products selectivity
increases from 9.7 to 61.9%. In contrast, the total current density remains mostly
unaffected by the modification once it is normalized with respect to the electrochemical
active surface area. Supported by a microkinetic model, we propose
that porous, thick films increase both local carbon monoxide partial
pressure and the carbon monoxide surface coverage by retaining in situ generated
carbon monoxide. This reroutes the reaction pathway toward multicarbon
products by enhancing carbon–carbon coupling. Our study highlights
the significance of customizing the molecular film structure to improve the
selectivity of copper catalysts for carbon dioxide reduction reaction.
