Breakthrough Study of Cell Signaling Holds Promise for Immune Research and Beyond
An international collaboration under the Center for Geometrically Engineered Cellular Systems has unraveled the inner workings of a process that allows hard-working T cells to tune out fake signals
For the first time ever, scientists have imaged the process by which an individual immune system molecule is switched on in response to a signal from the environment, leading to the critical discovery that the activation process involves hundreds of proteins suddenly coming together to form a linked network through a process known as a phase transition.
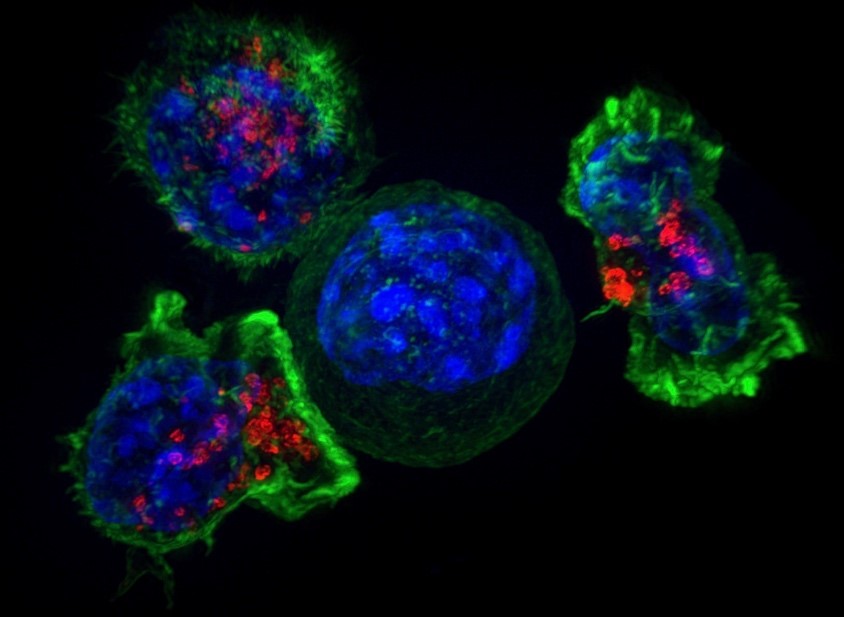
The new work, described in a paper recently published in Science, provides an enormous leap forward in our understanding of how the immune system is finely tuned to detect even a single virus molecule amidst a sea of millions of other molecules, allowing us to quickly recover from viral infections such as the flu. By learning how these particular proteins work, scientists will also have a better grasp on why their activity sometimes goes awry – events that can lead to autoimmune diseases, such as diabetes or rheumatoid arthritis – and may provide unique insights into how to direct a cancer patient’s own immune system to cure cancer.
“This is something that happens inside a living cell during the process of the cell making a decision – signal transduction is what we call it – and it’s how cells ‘think’ with chemical reactions,” said study leader Jay Groves, a professor of chemistry at UC Berkeley and a faculty chemist in the Lawrence Berkeley National Laboratory. “In the field of biology as a whole, the idea of a protein condensation phase transition has gained a lot of attention recently. Many groups all over the world study these phenomena, but until now, nobody knew how or why the cell is using them. “Our paper is, I believe, the first to directly test and confirm how a phase transition can regulate signaling,” Groves said. “And the big discovery is that it’s a molecular timing mechanism. The cell is using time to distinguish a genuine receptor stimulation from background chemical noise.”
This work is part of a larger international collaboration under the Center for Geometrically Engineered Cellular Systems, involving researchers from the University of Copenhagen, Berkeley Lab, UC Berkeley, and UC San Francisco. Center leader, Prof. Dimitrios Stamou from the Department of Chemistry at University of Copenhagen, comments: “These findings are of tremendous potential impact because similar mechanisms are likely involved in many other cell responses. We, for example, are currently investigating their involvement in the signalling of G protein coupled receptors, the proteins targeted by about half of all drugs in the market today.”
The research was funded by grants from the National Institutes of Health’s National Cancer Institute and the Novo Nordisk Foundation.
Contacts:
- University of Copenhagen: Prof. Dimitrios Stamou, stamou@chem.ku.dk
- University of California Berkeley: Prof. Jay Groves, JTGroves@lbl.gov & Press officer Aliyah Kovner
